Abstract
Background
Pembrolizumab, an antibody that blocks programmed cell death protein 1 (PD-1), has been FDA approved for several solid tumors and hematologic malignancies. Currently, pembrolizumab is under investigation for acute myeloid leukemia (AML) in combination with hypomethylating agents. It is established that AML is highly responsive to immunotherapy, as seen with the anti-leukemic effect of allogeneic hematopoietic stem cell transplantation (alloHSCT). However, response to cytotoxic T lymphocytes (CTLs) that target leukemia-associated antigens (LAAs) has been less reliable in eradicating disease. This insufficient response to LAA-specific CTLs is likely partially accounted for by the immune dysregulation seen in AML. Because of promising murine data that blockade of the PD1/PD-L1 pathway enhances the graft versus leukemia effect of alloHSCT, we investigated if adding pembrolizumab to CTLs that target the two LAAs CG1 and PR1 will enhance CTL antileukemia activities. CG1 and PR1are two HLA-A2 restricted nonameric peptides that we validated as promising AML targets derived from cathepsin G (i.e. CG1), and proteinase 3 (P3) and neutrophil elastase (NE) (i.e. PR1). We hypothesized that pembrolizumab added to CG1-CTLs and PR1-CTLs, will enhance their anti-leukemic effects with minimal off target toxicities.
Methods
Using a standard calcein AM in vitro cytotoxicity assay, we co-cultured AML targets, including U937 HLA-A2 + (U937-A2) AML cell line and primary patient HLA-A2 + AML samples, with CG1-CTL and PR1-CTL. AML cells were loaded with calcein AM and then incubated with CG1- and PR1-CTLs at increasing effector to target ratios. Pembrolizumab or isotype antibody were added to the cultures. After 4 hours, calcein AM was measured to determine cell viability. T2 cells pulsed with PR1 or CG1, were used as a positive control and non-pulsed T2 cells were used as negative control cells. For in vivo experiments, we used a human established AML xenograft mouse treatment model to determine the effect of pembrolizumab when added to CG1- or PR1- CTL in vivo. CG1-CTL and PR1-CTLs were expanded from the same donor and used as the effector cells. NOD/SCID gamma (NSG) mice (4-6-week-old females) were engrafted with AML samples by tail vein injection. After confirming engraftment, CG1- and/or PR1-CTL were administered to mice and pembrolizumab or isotype antibody [100ug/mouse] were given three times over two weeks. Mice were monitored for clinical GVHD and AML three times/week. Mice were sacrificed at approximately two weeks following treatment. To assess for GVHD, mouse tissues including spleen, liver, kidney, BM, intestine, brain, heart, and lung were harvested and fixed in 10% formalin. The fixed tissue samples were embedded in paraffin, sectioned, and stained with hematoxylin and eosin prior to histologic examination. Bone marrow (BM) was processed using standard methodology and analyzed for residual AML by flow cytometry.
Results:
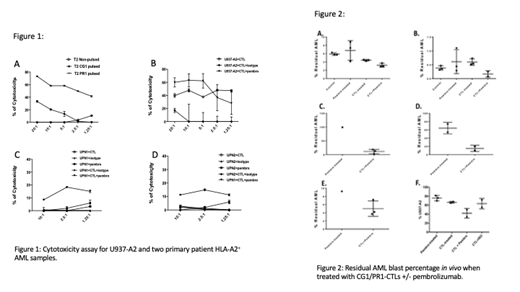
In vitro our data demonstrate that U937-A2 and two primary patient HLA-A2 AML samples, have enhanced cell lysis when treated with pembrolizumab (CTL+ pembrolizumab) in comparison with isotype (Iso) + CTL, pembrolizumab only, iso only or CTL only groups (Figure 1). In vivo data show a decrease in U937-A2 disease burden and primary patient AML after treatment with CG1and/or PR1-CTL (CTL-treated) . This decrease was enhanced when pembrolizumab was added to the CTL-treated mice (CTL+ pembrolizumab) in comparison with mice treated with pembrolizumab only (pembrolizumab-treated), or CTL only (CTL-treated) (Figure 2). Pathology data to assess toxicity in mice treated with CTL +/- pembrolizumab showed an enhanced pulmonary (perivascular and parabronchial) lymphocytic infiltration in mice treated with the combination of CTL and pembrolizumab. The other organs investigated, showed no change when combination therapy was used.
Conclusion:
We have validated in vitro and in vivo the enhanced killing of AML (cell lines and primary patient samples) by CG1-CTL and/or PR1-CTL after addition of pembrolizumab. Toxicity data show mild enhancement of lymphocytic infiltrate in the lungs after addition of pembrolizumab to CTL. Our data suggest that the strategy of combining LAA-specific CTL with immune checkpoint blockade could prove beneficial in the setting of adoptive T cell therapy and allogeneic stem cell transplantation for AML.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal